Elements, the simplest forms of matter, were made into the periodic table by Dimitri Mendeleev. The periodic table, which we are very familiar with due to chemistry lessons, contains various information. In this article, we have listed the periodic table features and interesting information on which dozens of studies have been made.
Periodic tableis a table that organizes chemical elements within the framework of certain rules. For many years, many scientists have worked on arranging the elements, but Dmitry Mendeleev He is known as the person who prepared the first version of the periodic table. Since then, the periodic table features have undergone various changes in light of developments in chemistry and physics.
Today, of 118 elements The periodic table on which it is located is considered one of the most important achievements in the history of science, with the information it contains and the conveniences it provides. Today, it has been a roadmap for many scientists for many years, which allows us to understand the material world more easily. periodic table We have covered all the details about it.
First, what is the periodic table?
The chemical elements often referred to as the periodic table periodic table, lists all chemical elements in ascending order of atomic numbers. Scientists use the periodic table to get some information about elements more quickly, such as atomic mass and chemical symbols. In addition, the order of the periodic table makes it easy to distinguish the details of many element properties, including electronegativity, ionization energy and atomic radius.
Many scientists have conducted various studies to order the elements in an orderly fashion. However, Dmitry Mendeleev in 1869 introduced the first version of the periodic table. Since then, the periodic table has evolved in the light of scientific advances.
So what are the features of the periodic table?

- Today it consists of 118 elements.
- As you go from left to right, the atomic number and mass number increase.
- As you go down from the top, the atomic number and metallic character increase.
- The first version of the periodic table had only 63 elements
- Elements in the same group have similar properties
153 years The periodic table, which has a history, contains numerous features. organized according to certain rules periodic tablegives information about many properties of elements. According to the first version prepared by Mendeleev in 1869, it has undergone many changes.
Interesting information about the periodic table:
- There are only 94 naturally occurring elements in the periodic table.
- Technetium is the first artificial element in the ruler
- The ruler is constantly revised by IUPAC
- The modern periodic table is arranged by increasing atomic number.
- Atomic size decreases as the atomic number increases in the periodic table
- Mendeleev correctly predicted the existence of yet undiscovered elements
- The atomic mass shown in the periodic table is the weighted average of all isotopes of the element.
- The periodic table is modeled after a card game.
There are only 94 naturally occurring elements in the periodic table.

Only elements in the periodic table 94 of themare elements found in nature. All other elements are man-made. Some sources state that more elements are formed naturally, because it is known that heavy elements can switch between elements when exposed to radioactive decay.
Technetium is the first artificial element in the ruler

technetiumin Italy in 1937 Emilio Segre It is an element discovered by The element with the atomic number 43 is an artificially produced is the first element. It has an atomic number of 43 and all isotopes are radioactive.
The ruler is constantly revised by IUPAC

The International Union of Fundamental and Applied Chemistry (IUPAC), as new developments in chemistry occur and new data become available. revise the periodic table is doing.
The modern periodic table is arranged by increasing atomic number.
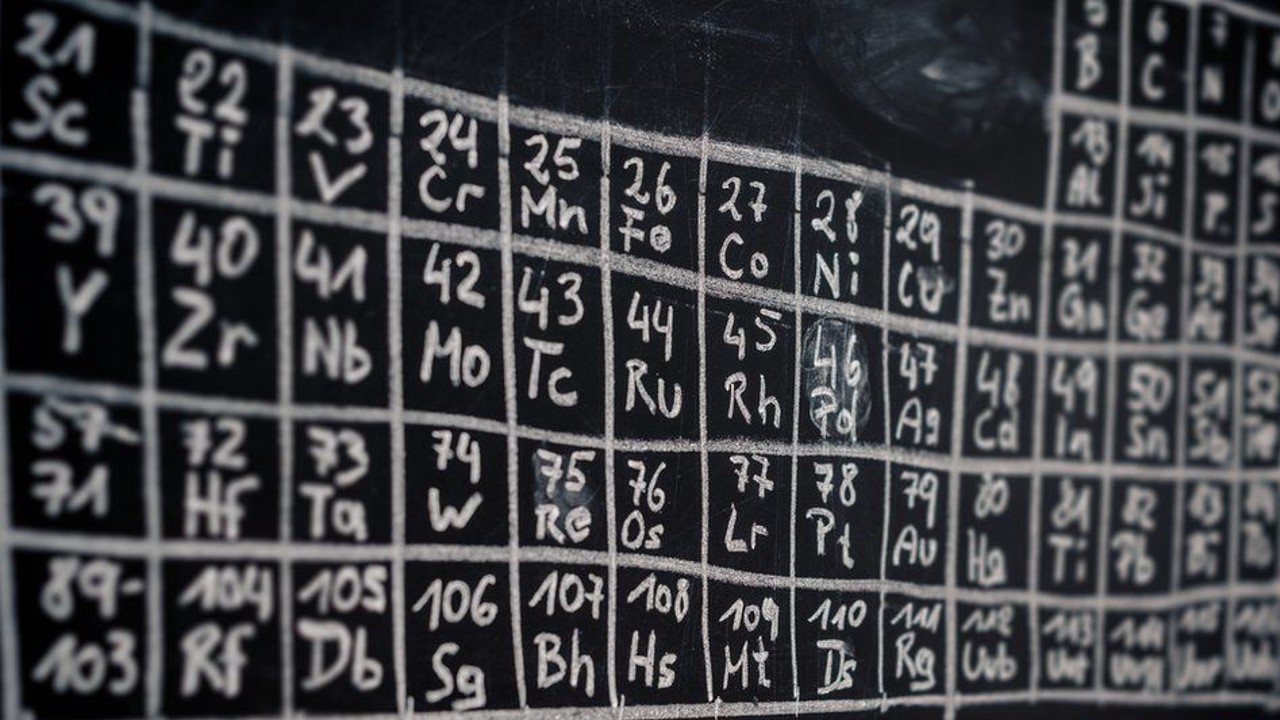
Mendeleev’s first periodic table The biggest difference between the modern periodic table and the Mendeleev elements to atomic mass while the modern periodic table is arranged according to the elements to atomic number regulation accordingly. For example, Hydrogen, the first element on the periodic table, has a proton and an electron. The second element, Helium, has two protons and two electrons. Each successive element has an extra proton and electron compared to the previous element.
Atomic size decreases as the atomic number increases in the periodic table

The atomic size of an element is expected to increase as the atomic number of its atoms increases, but this is not always the case. The size of an atom is determined by the diameter of its electronic shell. Therefore, as the element moves from left to right along a row, size of atoms usually shrinks.
Mendeleev correctly predicted the existence of yet undiscovered elements
Dmitry Mendeleev, is the scientist who prepared the periodic table in the most similar way to today’s periodic table. By arranging the elements according to their atomic masses, he noticed patterns of repetitive properties and grouped these elements together among themselves. When Mendeleev completed the first version of the periodic table, there were many elements and spaces that had yet to be discovered. Accurately identify these missing elements with various analyzes. to guess succeeded.
The atomic mass shown in the periodic table is the weighted average of all isotopes of the element
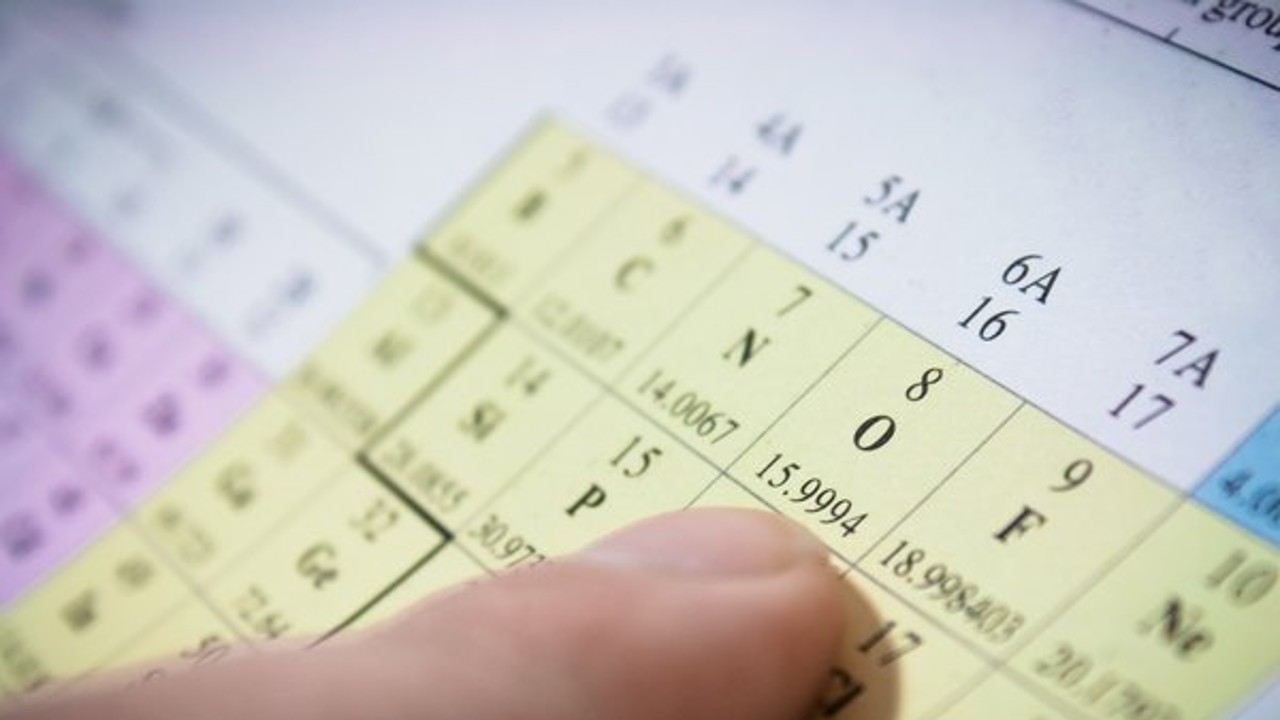
IsotopesAtoms with the same number of protons but different number of neutrons. Since the mass of an atom comes from its protons and neutrons, if the number of neutrons is different, each atomic mass of the isotope it will be different. Thus, the atomic masses you see in the periodic table are actually the weighted average of all isotopic masses.
The periodic table is modeled after a card game.

Known for his fondness for card games Mendeleev, He took these games as an example while preparing the periodic table. He wrote down the weight of each element on a separate card and put them all we know. solitaire sorted in the style of the game.
