The US group expects the first approvals shortly.
(Photo: dpa)
Dusseldorf The US pharmaceutical company Pfizer Tuesday presented mixed results from a study on the effectiveness of its planned anti-corona pill. Most important positive finding: In the study, taking the tablet saved 90 percent of high-risk patients from hospitalization and death. This refers to people with serious previous illnesses such as diabetes and cancer, for whom there is a high risk of death from Covid 19 disease.
Just as important: Initial laboratory results indicate that the agent is also effective when infected with the new virus mutant Omikron. At least 70 percent protection against hospitalization was achieved in patients with normal risk and no previous illness.
However, the US pharmaceutical company did not achieve another goal in the study: taking the pill should also reduce the symptoms of the disease in patients. The study data does not yet provide any evidence for this, but it is to be continued. As a result, Pfizer shares fell slightly before the market.
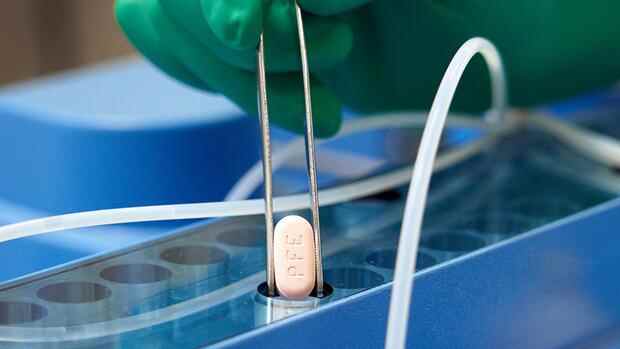
The anti-corona pill Paxlovid should be taken every twelve hours for five days shortly after the onset of symptoms. It is a combination therapy, because at the same time the patient is given an older antiviral agent.
Top jobs of the day
Find the best jobs now and
be notified by email.
Pfizer assessed the study results as positive overall. “We’re talking about an incredible number of lives saved and hospitalizations prevented. And of course we can drastically reduce the transmission if we use this agent quickly after infection, ”said research director Mikael Dolsten.
Approval of the agent in the USA and Europe is still pending. Pfizer could soon benefit from the coronavirus pandemic fight in two ways. The vaccine against Covid-10 developed jointly with the German company Biontech has already been sold billions of times.
New findings on Omikron in South Africa
On Tuesday, new data on the effect of the Biontech / Pfizer vaccine in the event of an infection with the currently rapidly spreading Omikron variant were also released. After that, two doses appear to offer significant protection against serious illness. This is the conclusion of a study by Discovery Health, South Africa’s largest private health insurance operator.
The underlying data comes from the first Omicron wave in South Africa between November 15 and December 7. During that time, Discovery Health recorded 211,000 positive test results, and 78,000 were infected with Omikron. It was found that those infected with a double Biontech vaccination had 70 percent protection against severe courses with hospitalization.
However, Discovery Health emphasized that this is only a preliminary result from the initial phase of the omicron dissemination. If the number of infections with the new variant increases significantly, the data could change. Overall, it has been shown that the risk of infection is higher with Omikron.
From the point of view of many experts, the appearance of new virus variants underscores the importance of the simultaneous development of new vaccines and drugs against Covid-19. It was already expected that the Pfizer pill would also be effective against an Omicron infection.
Because the drug paxlovid starts at a different point in the organism than the mRNA vaccines. The latter feed a blueprint into human cells, which is then used to produce parts of the spike protein from the coronavirus. This triggers an immune defense reaction that prepares the body for a real infection.
The anti-corona pills, on the other hand, target enzymes that control the replication of viruses in the cells. They work regardless of how strongly the spike protein has already mutated.
Read more about the fight against Corona
Another advantage is that the remedies can be taken as tablets and are therefore also suitable for household use under medical supervision. The antibody therapies available to date can practically only be administered in hospitals. But then it is often too late because the patient’s disease has progressed too far.
It is hoped that Covid patients will be able to take the new active ingredients for five days quickly after the onset of symptoms, thereby significantly reducing the risk of severe disease. Waves of infection could thus be better controlled, the number of hospital admissions and deaths could be noticeably reduced.
Pfizer manager Dolsten is expecting an emergency approval of the drug from the US FDA and other authorities in the EU and the UK soon. The US government has already secured ten million treatment units for more than five billion dollars.
The USA, like Germany, have also ordered larger quantities of the antiviral pill molnupiravir from the pharmaceutical company Merck & Co, which has also applied for emergency approval for the drug. In principle, it works in a similar way to the Pfizer remedy. However, in the clinical study with high-risk patients, it demonstrated lower protection against hospital stays and death.
More: These are the bearers of hope among corona drugs – and that’s how they work