Iron, aluminum, platinum, nickel, mercury, zinc, magnesium… Think of all these metals. Aren’t they all silver? However, the gold element, which is in the metal category, is not like that. But why?
One of the most striking features of gold, which is the symbol of luxury, wealth and splendor, is that it is bright yellow. Considering other metal elements gold has a different appearance Really interesting.
So what could be the reason why gold stands out from many other metals and takes on a different color? A journey dating back to Einstein’s theory Let’s go out and explain.
Let’s start with why metals are silvery.
Metals are generally substances that conduct electrons freely. When light strikes the metal surface, it is absorbed by the electrons on the surface and is of the same wavelength, i.e. same color spreads. Therefore, the fact that the metal is mostly metallic gray or silver is due to the unique properties of the metal. Some metals, such as bronze or gold, can be different colors. Let’s explain the reason through the gold element.
The electron configuration of metals can give them different properties.
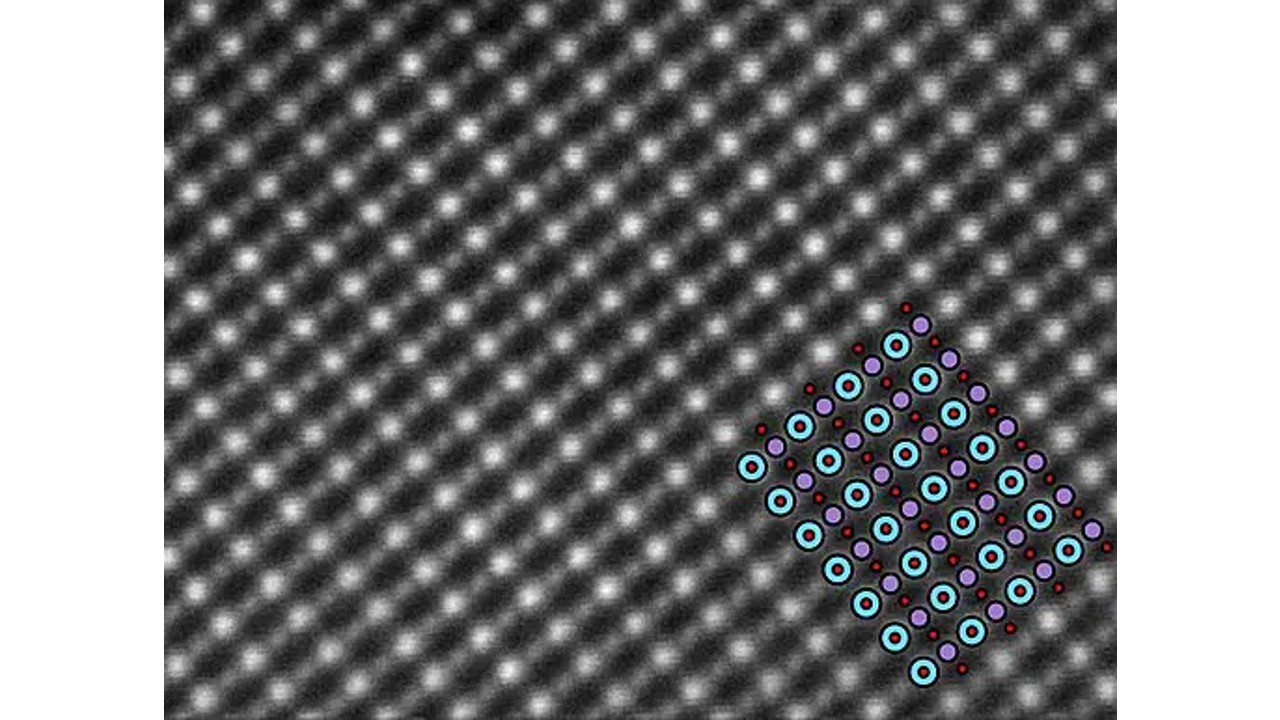
Each metal has a unique electron configuration, and these configurations determine the optical properties of the metal. Gold, known for its characteristic color yellow, is not silver like other metals because gold electron structure different.
Electrons moving freely outside the gold atoms, It absorbs light and emits it back, creating the yellow color. So, what kind of process is underneath and why is the color different?
Gold atoms are moving faster than we thought.
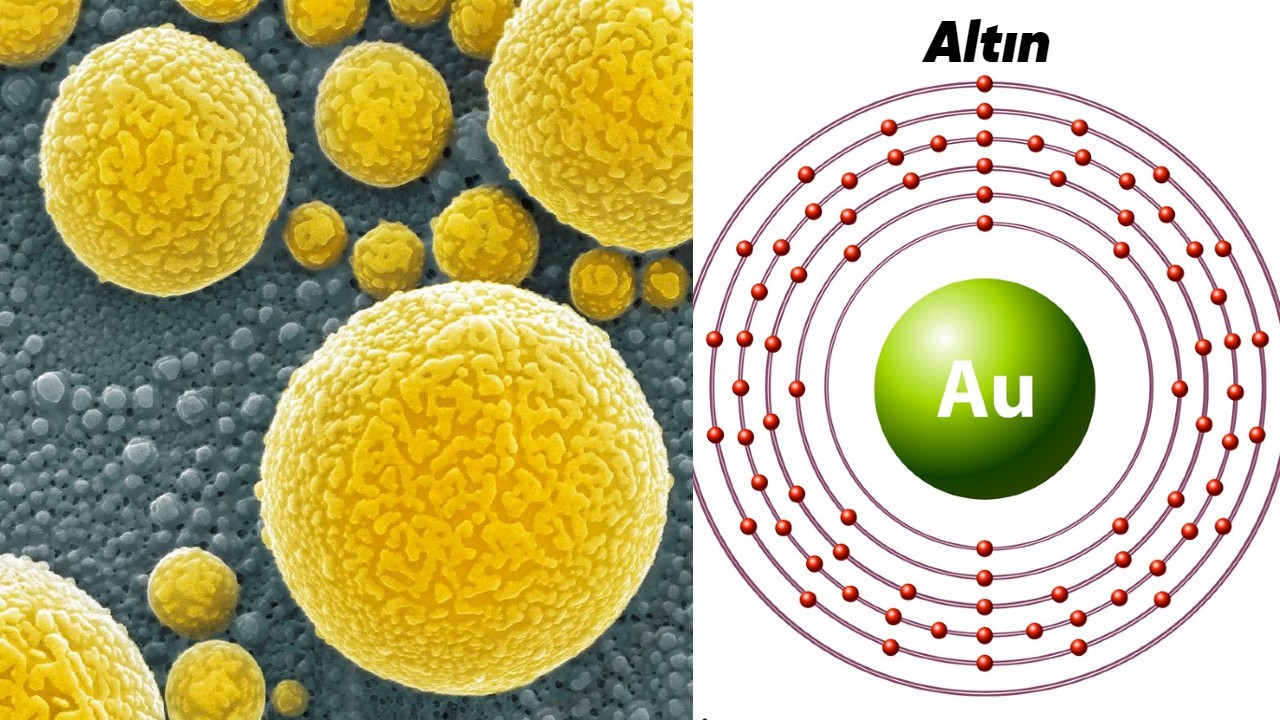
The relatively large gold atoms move at high enough speeds to create relativistic effects. This speed is so fast that it is close to the speed of light. relative speed, causing electrons to contract and masses to increase It affects the way it absorbs and reflects light. It shifts the wavelength of light absorbed by gold towards the blue end of the spectrum.
Gold absorbs blue light and reflects its opposite color in the spectrum, yellow, thus producing yellow, which has now become a symbol of luxury. Moreover, this feature From Einstein’s special theory of relativity and is due to different electronic transition energies in gold atoms, influenced by the binary nature of electrons.
What does Einstein’s theory of relativity have to do with it?
The properties of gold are mainly determined by “relative effects”. Relativity effects, Albert Einstein From the formula “E=mc2” stems from. Due to the attraction of the 79 times positively charged gold nucleus, the negatively charged gold electrons reach high speeds close to the speed of light (c), and the kinetic energy (E) cannot significantly increase their speed.
Instead, electrons increase their mass (m). Gold compounds have a higher chance of activating the triple bond between two carbon atoms. The atomic behavior of gold, although silver is its direct neighbor in the periodic system It is more similar to copper than silver is showing.
To summarize:

The reason why metals such as silver and gold have different colors is the electron behavior that arises from their atomic structure. Silver color, light from free electrons on the surface of the metal The yellow color of gold is due to its absorption and emission, while gold’s electrons are in different energy levels.
Our other content that may interest you:
RELATED NEWS
Aren’t the Gold-Plated Dishes That The Rich People Show Up To Our Eyes Harmful To Health?
RELATED NEWS
Why Is Gold Particularly Made Into “Ingot” Shape? Wouldn’t It Be Easier If We Made It Into Cubes?
RELATED NEWS
