The US group expects the first approvals shortly.
(Photo: dpa)
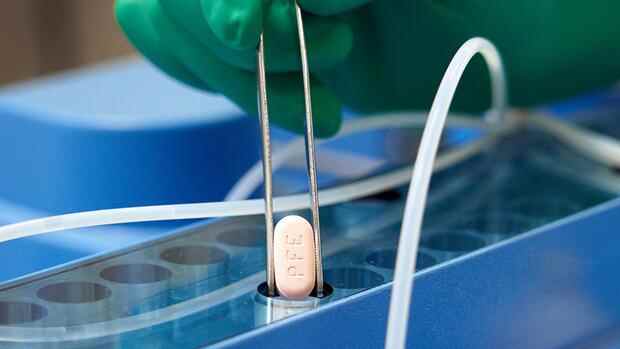
new York The US pharmaceutical company Pfizer sees the high effectiveness of its anti-corona pill confirmed after a final evaluation of the clinical study. Accordingly, the tablet showed an effectiveness of almost 90 percent in preventing hospital stays and deaths in high-risk patients, as Pfizer announced on Tuesday. The latest laboratory tests also indicated that the drug would also maintain its effectiveness against the fast-spreading omicron variant of the coronavirus.
Pfizer published preliminary study results in November that the pill was 89 percent effective in preventing hospitalizations or death compared to a placebo. The interim results were based on data from around 1200 people, the final results include a further 1000 test persons. None of the study participants who received Pfizer treatment died – compared with twelve deaths among those who took the placebo.
The drug company also released initial data from a second clinical study showing that the treatment reduced hospital stays by about 70 percent in around 600 adults at normal risk of infection. “We’re talking about an incredible number of lives saved and hospitalizations prevented. And of course we can drastically reduce the transmission if we use this agent quickly after infection, ”said Pfizer research director Mikael Dolsten.
The pill is given in combination with an older antiviral called ritonavir. The combination treatment with the brand name Paxlovid is taken every twelve hours for five days shortly after the onset of symptoms.
Top jobs of the day
Find the best jobs now and
be notified by email.
Pfizer manager Dolsten expects an emergency approval of the drug from the US FDA and other authorities, for example in the EU and the United Kingdom, in the near future. The US government has already secured ten million treatment units for more than five billion dollars.
No oral antiviral treatments for Covid-19 have yet been approved in the United States. The pharmaceutical company Merck & Co has also applied for emergency approval for its antiviral pill molnupiravir. However, this drug was only able to reduce hospital stays and deaths by about 30 percent in a clinical study with high-risk patients.
More: These are the bearers of hope among corona drugs – and this is how they work