You may have seen in some videos and images that an element called gallium shatters metals such as aluminum and steel, which are known to be very durable. So what about the fact that such a seemingly powerful element does not harm the human body?
The moment you hold gallium in your hand, It becomes slippery and slips through your fingers as if it were any liquid. It leaves only a sticky feeling and small stains behind.
Well, while gallium has the power to almost destroy many substances, How can it be explained that it does not harm the human body?
Here liquid gallium was left on the computer We see. Molten gallium shatters the computer after a certain period of time.
https://www.youtube.com/watch?v=1tn5QhUSMjQ
In the previous video, it was gallium that could tear the computer apart, here it is It never harms the hand.
https://www.youtube.com/watch?v=J5TTiNkgwXs
So how does this situation occur?
In fact, gallium causes metals such as aluminum and steel to melt. chemical reaction caused by the entry. Just as water causes iron to rust but does not harm our hands, a more accelerated version of a similar situation applies to the interaction of these elements (gallium, aluminum).
When this element combines with aluminum, it forms an alloy called amalgam. This alloy seeps into the atomic crystal structure of aluminum and dissolves it, making it brittle. Thus, solid aluminum can easily break down when combined with gallium, and gallium It causes structural damage to the substance it interacts with.
The surprising thing is that for gallium to achieve this, It requires very little intervention. Even placing a very small amount of gallium on an aluminum and steel material is sufficient for the alloy to form.
Silver-coloured gallium has a very low melting point of 29.4℃. It alloys easily with most metals and can be used as a component even in low melting point alloys.

This element melts and becomes liquid quite easily, but very high temperatures are required for it to boil. It expands when frozen and is only one of the few substances that can do this.

In addition, it is seen that the alloy obtained as a result of the combination of metals such as gold, nickel and copper with gallium hardens at mouth temperature, and this alloy is It is used as a filling material in dentistry.
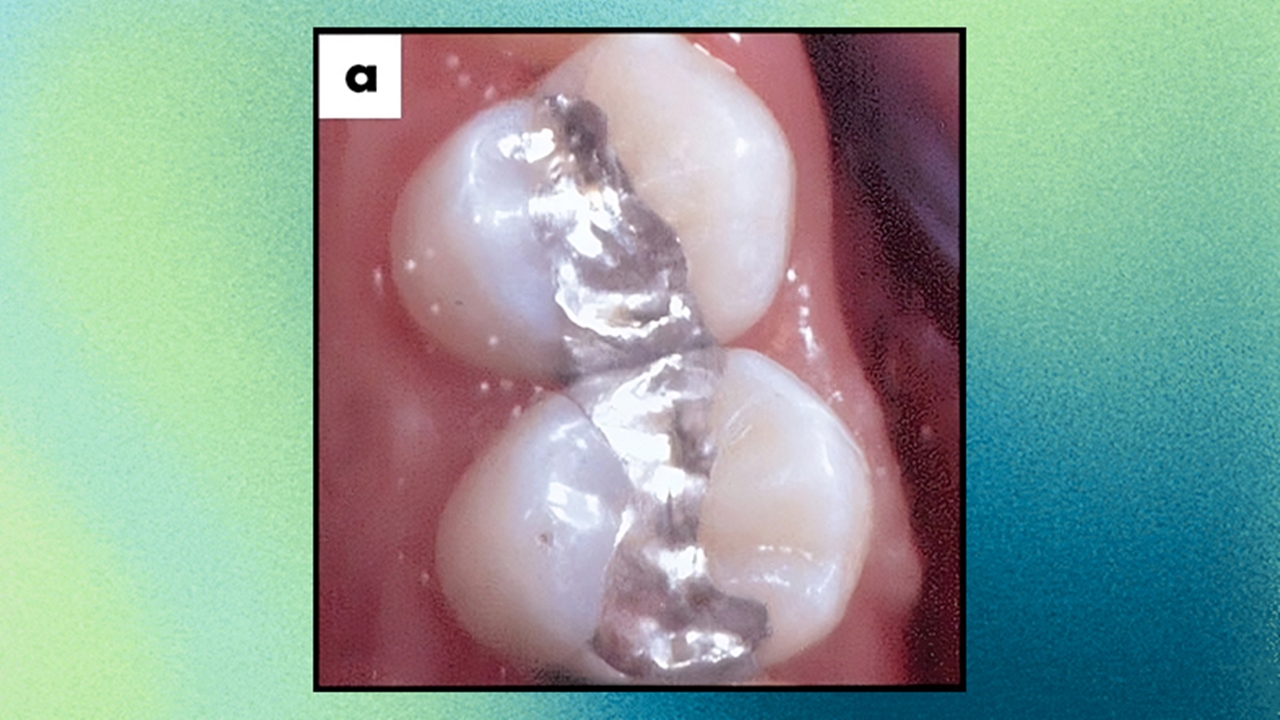
Playing with or handling gallium, which is a 100% metal, does not pose any danger. In addition, the toxicity of the compounds contained in this element is quite mild, but of course they should not be inhaled or swallowed.
In summary, gallium takes liquid form in our hands, but it damages different metals. It is the result of a chemical reaction with metals. For example, when we come into contact with water and salt separately, nothing happens, but when these two compounds come together, the salt dissolves in the water.
Well, gallium is for you too. Does it remind you of the movie Terminator 2?
RELATED NEWS
We Remember From Chemistry Lessons: What is the ‘Atomic Number’ of the Element, Determined Based on the Number of Protons, and How to Find It?
RELATED NEWS
