In the United Kingdom, authorities have approved the use of a CRISPR therapy called Casgevy to treat two inherited blood diseases. So what is CRISPR and why is this decision important?
For the first time in the world in the treatment of diseases CRISPR The use of gene editing technology was permitted. The first technology to receive this permission, casgevy It became known as Exa-cel.
on November 16 UK Medicines and Healthcare products Regulatory Agency (MHRA) The permission given by will be used in the treatment of sickle cell anemia and transfusion-dependent Mediterranean anemia. Previously, the US Food and Drug Administration had declared that the drug was safe for clinical use, but did not allow it to be used in treatments. These permits were expected to be approved in December 2023.
A new era may begin in gene therapy
The MHRA’s decision is historic. After this decision gene therapy As a much more popular and accepted method, it will be usable in the fight against many hereditary diseases. However, the decision raises many questions that need to be answered regarding sustainability, cost and long-term impacts.
casgevy It will first be used in the treatment of sickle cell anemia and transfusion-dependent Mediterranean anemia. In the first of these diseases, the red blood cells are not round and are sickle-like It happens in a structure. These cells have problems such as getting stuck on each other and blocking the vessels, causing pain and a feeling of pain, and short lifespan. If care is not taken, this disease can cause serious consequences.
In Mediterranean anemia, the number of red cells in the blood is very low. In transfusion dependent Mediterranean anemia This production is so low that patients need regular red cell transplants to survive. Gene editing seems to be an important alternative for the treatment of these genetically occurring diseases.
How does CRISPR work?
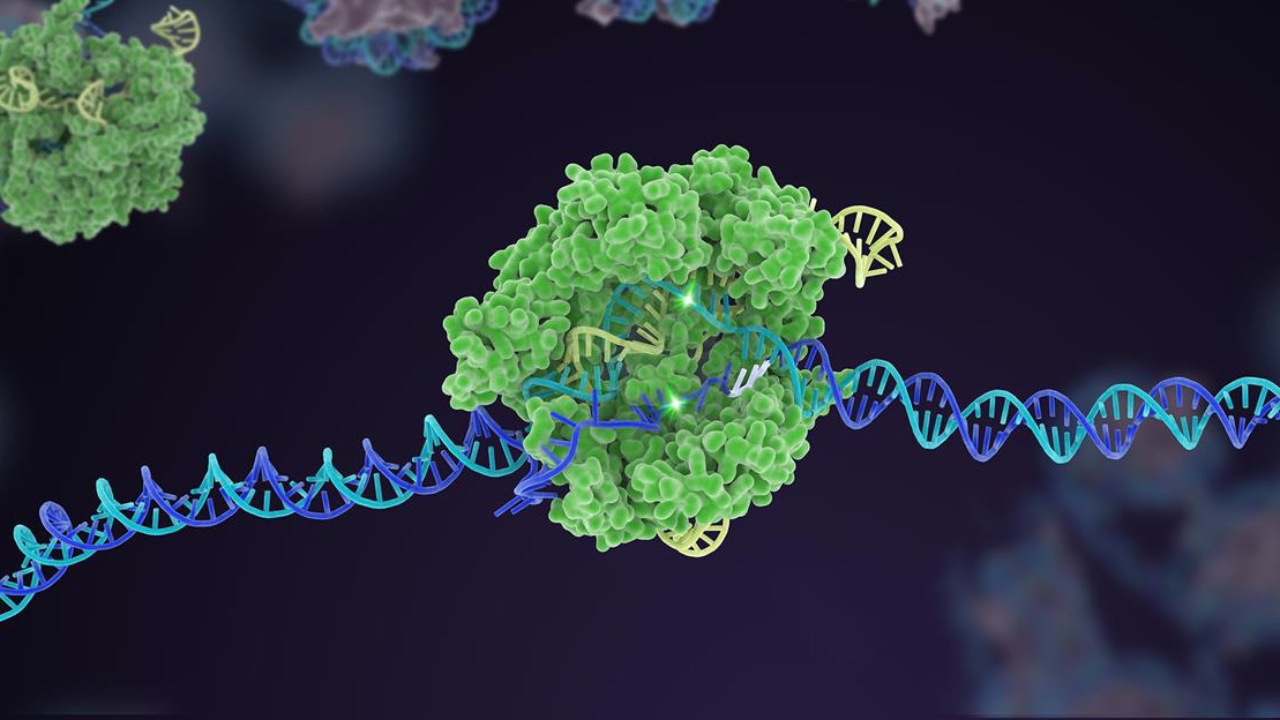
First appeared in 2012 CRISPR The technology actually has a very simple working logic. In essence, CRISPR is used as ultra-precise genetic scissors. DNA a suitable one for the part to be replaced. RNA It is synthesized and combined with an enzyme called Cas9. The part containing the faulty code from the double-stranded DNA is cut out and the edited version is added instead. In other words, if we consider people as digital products, some of the codes in our source software can be cleared of errors in this way.
Casgevy also targets a gene called BCL11A. This gene is responsible for the production of hemoglobin under normal conditions. from fetal version It synthesizes a protein that enables it to transition into the adult version. However, in sickle cell anemia and Mediterranean anemia, adult homoglobins It becomes problematic. With the discontinuation of BCL11A, hemoglobins can continue to be used as they were at birth.
RELATED NEWS
A Way Has Been Found to Make Cancer Cells “Self-Destruct”
On the other hand, this process will not be easy at all. People may need to be hospitalized for about 1 month for all hemoglobin levels to gradually change and red cell formation in the bones to reach the desired level. Also some medicines immunosuppressants will be taken together.
Still, 28 of 29 sickle cell anemia patients who participated in clinical testing for more than a year He did not experience a pain attack. 39 of 42 Mediterranean anemia patients did not need red cell supplementation during the same period. The need for blood supplements in the remaining three patients was reduced by 70%.
Genetic editing raises safety concerns.
In the eyes of many, genetic editing is an important weapon in the fight against disease. However, there are some general concerns about this issue.. Imperial College LondonDavid Rueda, Head of the Department of Molecular and Cellular Biophysics atIt is known that CRISPR can have unknown consequences on cells receiving treatment. “It may be imperative to see sequencing data for the entire gene to reach a conclusion.” He uses the expressions. this too CRISPR It necessitates the examination of all DNA against unexpected effects of the treatment.
Casgevy’s It is not yet known when it will be available to patients. The ability to use the treatment depends largely on knowing its costs. Let’s say that gene therapy can cost millions of dollars. Developing the drug Vertexhas not announced a price yet, but the company continues to negotiate with the authorities to ensure that the drug reaches patients and is covered by insurance.
Source :
https://www.livescience.com/health/genetics/the-worlds-1st-crispr-therapy-has-just-been-approved-heres-everything-you-need-to-know